Water Quality Research Group
Professor Pirbazari's Research Group
Research Activities
Heavy metal contamination of drinking water and groundwater sources continues to be a growing problem gaining attention from regulatory bodies. Federal and state environmental agencies have proposed regulations requiring removal of metals to low ppb levels. Conventional methods are unable to meet these standards. Our research group intends to develop removal technologies capable of meeting these new standards.
Utilizing chromium as a model, novel biological treatment systems facilitating metal removal are under development utilizing dissimilatory metal reducing bacteria (DMRB). Dedicated, low-detection-limit analytical instruments are employed by the water research group to ensure metal removal to below ppb levels. Kinetic screening studies with DMRB have been performed to screen for candidates capable of rapid reduction under various operating conditions. Following selection, different reactor systems (such as batch and continuous flow systems) will be used for optimization of operating conditions and determination of biokinetic parameters.
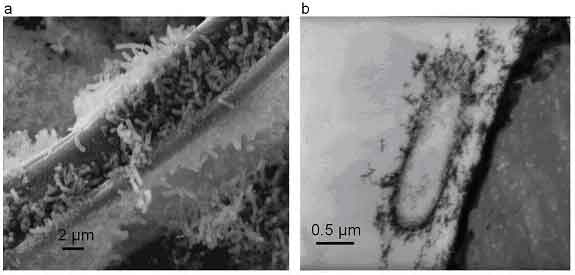
Figure 1. Immobilized biomass of metal reducng bateria imaged by (a) SEM and (b) TEM techniques
One aspect of this research involves the development of in situ and ex situ treatment technologies in collaboration with the microbiology research group led by Professor Ken Nealson. For ex situ applications, the novel technology will be incorporated cost-effectively into current waste streams and reactor systems. This method will provide additional removal capacity for heavily contaminated waste streams to allow for regulatory compliance prior to discharge. In situ treatment methods are also in development which will allow for treatment systems to be inserted into existing groundwater basins (in either vadose or saturated zones). This provides a noninvasive method of sequestering contaminants from potential drinking water sources in addition to providing the potential for recovery and reuse of useful species.
The Metropolitan Water District of Southern California (MWD) and its partners recognize that a major source of their water is Colorado River water (CRW). The CRW typically contains high total dissolved solids (TDS) concentrations with the 90th percentile level of 703 mg/L. The TDS levels could increase further if droughts were experienced in the Colorado River watershed. The Desalination Research and Innovation Partnership (DRIP) program was initiated by MWD and its partners to develop large-scale and cost-effective desalination technologies for the treatment of CRW and other water sources containing high levels of TDS. The DRIP program chose reverse osmosis for its proven reliability and potential for large-scale applications. A major problem associated with large-scale reverse osmosis processes is the minimization and disposal of brine or concentrate, which is a concentrated mixture of inorganic salts and organic matter. A full-scale desalination plant necessarily incorporated conventional pretreatment followed by split-flow treatment, wherein a third of the flow was subjected to reverse osmosis.
Owing to the large-scale operations of conventional treatment plants by the MWD at 320-750 million gallons per day (MGD), the desalination plants require high operation capacities. The ultimate goal of reducing the TDS to 500 mg/L was to be met by split-flow treatment, wherein one-third of the CRW flow would be treated by reverse osmosis. Even so, desalination plants of capacity exceeding 150 MGD were required for meeting the large size of the MWD treatment plants. Prior estimates from the DRIP Program show that a typical 150-MGD desalination plant, at a low recovery of 85%, would generate 22 MGD of brine. The disposal of brine in inland facilities presented a major problem owing to the large volume generated; marine disposal not being considered as a viable option due to poor access to the ocean. High recoveries of over 95% in the reverse osmosis plants were therefore desirable for reducing the intensity of the brine disposal problem. Minimization of brine concentrate volumes using secondary brine-concentration processes resulted in lower area requirements for drying beds in inland facilities that do not have access to the brine disposal line. A number of technologies were considered and reviewed with reference to brine concentrate minimization including several thermal and non-thermal processes. Thermal technologies involving phase changes such as distillation processes and crystallizer processes used by the paper-pulp and oil industry were considered very energy intensive.
Reverse osmosis appeared the most economically viable technology for this purpose. A major obstacle to operating reverse osmosis processes under a high-recovery scenario was the precipitation of sparingly soluble inorganic salts present in the brine such as barium sulfate, calcium sulfate, strontium sulfate, and calcium carbonate, as well as silicate salts. The precipitation of these salts could be controlled at low recoveries (below 90%) by the use of anti-scalants and pH control. However, at high recoveries (over 90%), anti-scalants were often ineffective, and pH adjustment was effective only for carbonate salts and relatively inefficient for sulfate salts (barium and calcium sulfates) or silicate salts that have low aqueous solubility and low pH dependence. Hence, removal of sulfate from the brine was required for reducing membrane fouling and maintaining high permeate fluxes during reverse osmosis. Biological sulfate reduction (BSR) appeared a viable option that requires investigation. The present project was geared towards the application of the BSR process for lowering sulfate concentrations in the aqueous phase, and simultaneously removing other organic components associated with membrane fouling problems.
Project Rationale
The reduction of sulfates by the biological sulfate reduction system and the treatment of the resultant hydrogen sulfide by a suitable technique effectively lowered the potential for the precipitation of barium sulfate, calcium sulfate, strontium sulfate, calcium carbonate, barium carbonate, and other inorganic scalants such as silicates, and reducing the fouling potential of reverse osmosis membranes during brine concentration and minimization. While precipitation of carbonate and silicate salts could be controlled by pH adjustment, precipitation of sparingly soluble sulfates could not be easily controlled by this technique. Under the circumstances, biological conversion of sulfate to sulfide in aqueous medium using the BSR technology was considered an efficient and cost-effective strategy. The hydrogen sulfide produced in the bioreactor was collected and fed into a gas-phase treatment system for conversion to innocuous products. The reduction in fouling potential of reverse osmosis membranes as a consequence of reduction in sulfate and other inorganic concentrations had to be evaluated. The bioreactor process and the reverse osmosis concentration and minimization of brine were optimized with reference to several operational variables at the bench-scale level, including the reactor configuration, type of solid support media, reactor hydraulic residence time, microbial population, and organic electron donors such as methanol, ethanol, acetate, etc., for sustaining the microbial population. This would minimize the pilot-scale studies required to validate the treatment strategy, and facilitate design and cost estimation of a full-scale BSR reactor system.
In the present treatment strategy, the reverse osmosis brine concentrate was treated in a bioreactor system for reduction of sulfates to prevent membrane scaling by precipitation. Anti-scalant was added to the bioreactor effluent before passage through a flat-sheet membrane test-cell for evaluating the fouling potential. The gas from the bioreactor system was passed through a biofiltration unit for the removal of hydrogen sulfide. Systems were used for analyzing the characteristics for the bioreactor effluent as well as to the influent and effluent of the hydrogen sulfide treatment system. An analytical system was employed for determining the fouling potential of reverse osmosis membranes during the treatment of process effluent from the BSR reactor system.
References
Samee, M., Jung, J., Vahdati, A., Ravindran, V., Williams, M.D., and Pirbazari, M. “Biological Sulfate Reduction of Reverse Osmosis Brine Concentrate: Fluidized Bed Adsorber Reactor Studies,” with Pirbazari, M., Samee, M., Jung, J., Vahdati, A., and Williams, M.D. AIChE Annual Meeting, Cincinnati, Ohio, Oct. 31-Nov. 4, 2005.
Jung, J., Samee, M., Vahdati, A., Ravindran, V., Williams, M.D., and Pirbazari, M. “Biological Sulfate Reduction of Reverse Osmosis Brine Concentrate: Batch Reactor and Chemostat Studies,” AIChE Annual Meeting, Cincinnati, Ohio, Oct. 31-Nov. 4, 2005.
Samee, M., Jung, J., Vahdati, A., Williams, M.D., Ravindran, V. and Pirbazari, M. “Microbial Reduction of Sulfate in Reverse Osmosis Brine Concentrate: Batch and Chemostat Reactor Systems,” with Jung, J., Samee, M., Vahdati, A., Williams, M.D., and Pirbazari, M, American Water Works Association Meeting, California-Nevada Section, Burlingame (San Francisco), California, April 24-26, 2006.
Samee, M., Jung, J., Vahdati, A., Williams, M.D., Ravindran, V. and Pirbazari, M. “Microbial Reduction of Sulfate in Reverse Osmosis Brine Concentrate: Fluidized Bed Adsorber Reactor Systems,” American Water Works Association Meeting, California-Nevada Section, Burlingame (San Francisco), California, April 24-26, 2006.
Pirbazari, M., Ravindran, V., Jung, J., Samee, M., and Vahdati, A. “Biological Sulfate Reduction for Recovering Reverse Osmosis Concentrate,” Final Project Report, 225 pp., submitted to the United States Environmental Protection Agency, and the Metropolitan Water District of Southern California and under the auspices of the Desalination Research and Innovation Partnership (DRIP) II, Grant Assistance XP-97933101-0, April 2006.
I. Membrane Transport Models for Membrane Processes
Membrane transport models are conceptualized and developed for predicting flux decline in membrane processes due to membrane fouling, concentration polarization and scaling phenomena. The models examine the transport mechanisms associated with these phenomena, and investigate permeate flux decline in ultrafiltration and nanofiltration processes. The modeling approach investigates two important aspects: predicting the permeate flux and removal of natural organic matter (NOM) as a function of time.
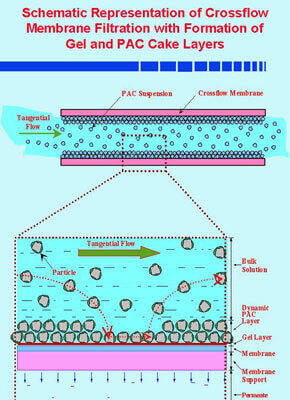
 The study investigates the formation of the gel layer, concentration polarization layer, and internal pore fouling that are mainly responsible for flux decline. The model equations involve the classical advection - diffusion transport equations with appropriate boundary conditions for the membrane filtration system. These equations incorporate the mass transfer resistances attributed to surface fouling, internal pore fouling, concentration polarization, and boundary layer effects depending upon the fluid dynamic regime. The model equations are solved by different types of finite difference schemes to optimize the computation accuracy and efficiency. The modeling approach could be applied to membrane systems, including reverse osmosis, nanofiltration, ultrafiltration, and microfiltration.
The study investigates the formation of the gel layer, concentration polarization layer, and internal pore fouling that are mainly responsible for flux decline. The model equations involve the classical advection - diffusion transport equations with appropriate boundary conditions for the membrane filtration system. These equations incorporate the mass transfer resistances attributed to surface fouling, internal pore fouling, concentration polarization, and boundary layer effects depending upon the fluid dynamic regime. The model equations are solved by different types of finite difference schemes to optimize the computation accuracy and efficiency. The modeling approach could be applied to membrane systems, including reverse osmosis, nanofiltration, ultrafiltration, and microfiltration.
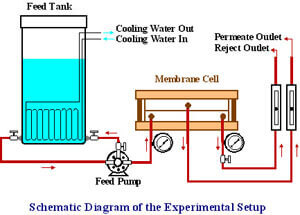
An important aspect of this research involves verification of the model by laboratory scale experiments performed for plate-and-frame nanofiltration and ultrafiltration membranes. The experiments are performed using model compounds to simulate different classes of membrane foulants. These model compounds represent organics with different structural and molecular weight characteristics, covering a wide spectrum of organic foulants encountered in water treatment. The experiments are conducted for different membranes because the surface characteristics of the membrane material determine the extent of fouling corresponding to different classes of foulants. In these studies, the permeate flux and the membrane rejection characteristics with respect to the model compounds are determined. The experimental results verify the validity of the model with regard to the temporal variation of permeate flux and permeate solute concentration. Also, real groundwaters are used to evaluate the model predictive capabilities. The following figure shows the experimental setup for the membrane performance tests.
II. Surface Characterization of Membranes
Surface properties of membranes are of critical importance in establishing their potential for fouling, and their performance with respect to permeate flux and solute rejection. Surface characteristics and membrane performance are related to the membrane material, membrane type, nature of feed solution, and interactions between membrane and solutes. In the present research, several methods for characterizing membrane surface properties are investigated. These methods include measurements of membrane sorption, zeta potential, contact angles, and electron microscopy techniques in combination with X-ray diffraction. These experiments are intended to facilitate a qualitative and quantitative evaluation of membrane sorption characteristics with respect to foulants, membrane surface hydrophobicity, and membrane surface charge under different experimental conditions. In particular, the electron microscopy studies in conjunction with X-ray diffraction facilitate the qualitative and quantitative evaluation of different types of foulants including inorganic foulants. An additional benefit of electron microscopy and X-ray diffraction studies, although of secondary importance, is the determination of surface morphologies of different types of fouled membranes in comparison with new and/or cleaned membranes.
Current methods for determining the adsorption affinity of membrane surfaces for natural organic matter (NOM) are not adequate to simulate the actual adsorption of membrane processes. A new technique is therefore developed for simulating the actual adsorption of NOM on membrane surface. Another important parameter in membrane sorption is the charge density on the membrane surface, which is related to the zeta potential. The widely used techniques for zeta potential determination involve measurements of streaming potential and electro-osmotic flow. These two techniques are inadequate for analyzing the actual zeta potential on an asymmetric membrane surface. In our technique, zeta potential of the membrane surface is measured directly by using a zeta-meter. Further, contact angle methods are used to measure the hydrophobicity and wettability of the membrane surface. In this study, the sessile drop method and captive bubble methods are employed.
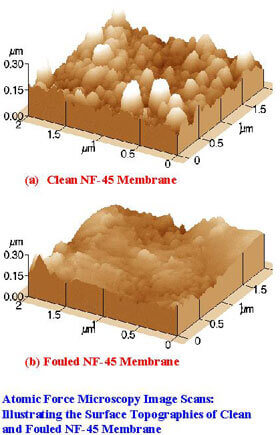
In these investigations, membrane surface characterization experiments are conducted for different types of membranes, and feed solutions containing different classes of foulants. The results from these studies would provide more specific information on the potential for membrane fouling for different types of membranes, membrane materials, and classes of foulants in the feed. The following atomic force microscopy images show the surface topographies of virgin membrane (a) and membrane fouled by tannic acid (b).
References
"Nanofiltration of Natural Organic Matter: Membrane Characterization," Tu, S.C., Tsai, H.H., Ravindran, V., and Pirbazari, M., (in preparation).
"A Pore Diffusion Transport Model for Performance Prediction of Membrane Processes in Water Treatment Applications," Tu, S.C., Ravindran, V., and Pirbazari, M., (in preparation).
"Predictive Membrane Transport Model for Nanofiltration Processes in Water Treatment," Tu, S.C., Ravindran, V., Den, W., and Pirbazari, M., AICHE Journal, June 2001.
"A Pore Diffusion Transport Model for Predicting Membrane Performance in Water Treatment Applications," Tu, S.C., Ravindran, V. and Pirbazari, M., Presented at the American Institute of Chemical Engineers Annual Meeting, Los Angeles, California, November 12-17, 2000.
"Evaluations of Membrane Fouling Potential in Water Treatment Applications," Tu, S.C., Ravindran, V. and Pirbazari, M., Presented at the Nation Conference in Environmental Engineering, American Society of Civil Engineers, Norfolk, Virginia, July 26-28, 1999.
"Surface Characterization of Nanofiltration Membranes in Water Treatment Applications," Tu, S.C., Ravindran, V., Tsai, H.H., and Pirbazari, M., Presented at American Institute of Chemical Engineers Annual Meeting, Los Angeles, California, November 16-21, 1997
"A Membrane Transport model for Predicting Permeate Flux in Nanofiltration Processes," Tu, S.C., Ravindran, V., Badriyha, B.N., and Pirbazari, M., Proceedings of the 1997 Membrane Technology Conference, pp. 487-498, American Water Works Association, Denver, Colorado, 1997.
"A Membrane Transport Model for Predicting Permeate Flux in Nanofiltration Processes," Tu, S.C., Pirbazari, M., Ravindran, V., Badriyha, B.N., and Pirbazari, M., presented at the 1997 Membrane Technology Conference, American Water Works Association, New Orleans, Louisiana, February 25, 1997.
Implementation of ozone into water treatment processes has proven effective in the reduction of disinfection by-products (DBP’s) and inactivation of pathogenic protozoa. Ozonation of waters having moderate to high dissolved organic carbon (DOC) fractions often results in the production of a myriad of compounds, the most abundant of which include aldehydes, ketones, ketoacids, and carboxylic acids, typically in the C2-C3 range. These species present environmental concerns of a toxicological nature regarding regrowth of potentially pathogenic migroorganisms in water distribution systems. As a result, biological filtration to remove these low molecular weight biodegradable compounds will most likely be mandated for water treatment processes employing ozone as a pre-oxidant.
A novel membrane-bioreactor process (MBR) has been developed for the removal of biodegradable dissolved organic fractions (BDOC) from drinking water, by integrating a cross flow microfiltration (MF) membrane with a suspended growth biofilm process. Removal of BDOC fractions is accomplished using a mixed culture of heterotrophic microorganisms. Microfiltration membranes provide a barrier to pathogenic protozoa (e.g. Cryptosporidium), bacteria, and viruses. To address the fouling issues in the MBR process, different cleaning techniques are being investigated.
Research efforts are also focusing on the development of a transient predictive model for the MBR process. At its core, the MBR model couples Monod growth kinetics with the homogenous surface diffusion model, and provides a temporal profile of substrate concentrations. This model will provide a powerful tool for process scale-up, as well as helping to evaluate the influences of critical process variables such as hydraulic residence time, biomass concentration, and carbon concentration.
Relevant Publications/Presentations
M.D. Williams, H.S. Tsai, and M. Pirbazari, "Membrane Bioreactor Process for Ozone Byproduct Removal From Drinking Water," In preparation.
M.D. Williams, H.S. Tsai, and M. Pirbazari, "Optimizing Biodegradable Organic Matter Removal From Drinking Water in a Membrane-Bioreactor Process," proceedings, AWWA Water Quality Technology Conference, Salt Lake City, UT, Nov. 2000.
M.D. Williams, H.S. Tsai, and M. Pirbazari, "Development of a Predictive Membrane-bioreactor Model for the Removal of Biodegradable Organic Carbon from Water," AIChE Annual Conference, Los Angeles, CA, Nov. 2000.
We have developed integrated technologies for water, wastewater treatment, and water reclamation and reuse. The hybrid process amalgamates membrane filtration, adsorption, and biological degradation for the treatment of industrial wastewaters of complex composition. The efficacy of this integrated process lies in the reduction of membrane fouling and concentration polarization phenomena that adversely affect the economic viability of membrane filtration.
We are utilizing this process for the purification of water contaminated with gasoline. The hydrocarbons of environmental concern include several volatile organic compounds (VOCs) such as benzene, toluene, ethylbenzene and xylene, and methyl-t-butyl-ether (MTBE), an important additive to gasoline. The hybrid process integrates a membrane filter (ultrafilter) and bioactive adsorbent such as powder activated carbon (PAC) with an immobilized biofilm layer. The technology integrates the advantageous features of membrane filtration, adsorption and biodegradation in one efficient and cost-effective process for treatment of contaminated waters. The ultrafiltration membrane sequesters the microbial population within the bioreactor unit, and further prevents the loss of microbial enzymes. The PAC adsorbent serves to mitigate the endemic problems of flux decline due to membrane fouling and concentration polarization. Other adsorbents besides PAC are being employed to promote biodegradation, but simultaneously prevent membrane permeate flux decline due to irreversible pore plugging (internal fouling), and gel formation (concentration polarization) attributed to organic foulants and biofoulants. This is accomplished by reducing the thickness of the boundary layer at the membrane surface, and promoting back-diffusion of potential foulants into the bulk liquid phase.
We are currently developing a mathematical model for performance prediction of the hybrid process which involves sorption, biofilm degradation, and bulk-fluid phase degradation of gasoline contaminants including MTBE on the bioactive adsorbent. The model predicts the extent of solute removal by adsorption and biodegradation mechanisms. Model verification is accomplished by laboratory scale experiments. The model essentially employs adsorption and biodegradation parameters determined by independent experiments. The adsorption equilibrium parameters are determined by completely mixed batch (CMB) reactor studies. Adsorption rate parameters including the adsorbent surface diffusion and film transfer coefficient are evaluated by CMB rate studies. Biological parameters that include the Monod constants, endogenous decay coefficient and microbial yield coefficient are determined from continuous flow biokinetic studies for the gasoline contaminants. The integrated process model will predict the removal of gasoline components using the above technology.
Relevant Publications/Presentations
"Integrated Membrane - Biodegradation Process for the Removal of Gasoline form Groundwater," Pirbazari, M., Ravindran, V., Williams, M., Bardiyha, B.,Tu, S.C., Tsai, H.H., and Walker, S.L. (in preparation).
"Membrane Fouling: Characterization and Control," Pirbazari, M, Ravindran, V., Williams, M., Tu, S.C., and Tsai, H.H. (in preparation).
"Nanofiltration of Natural Organic Chemicals: Membrane Fouling Characterization," Pirbazari, M., Ravindran, V., Badriyha, B., Tu, S.C., and Tsai, H.H. (in preparation).
"A Membrane Transport model for Predicting Permeate Flux in Nanofiltration Processes," Pirbazari, M., Ravindran, V., and Badriya, B.N., and Tu, S.C., in Proceedings of 1997 Membrane Technology Conference, pp. 487-498, American Water Works Association, Denver, Colorado, 1997.
"A Membrane Transport Model for Predicting Permeate Flux in Nanofiltration Processes," Pirbazari, M., Ravindran, V., and Badriya, B.N., Tu, S.C., presented at the 1997 Membrane Technology Conference, American Water Works Association, New Orleans, Louisiana, February 25, 1997.
"Hybrid Membrane Filtration Process for Leachate Treatment," Pirbazari, M., Badriyha, B., Kim, S.H., and Ravindran, V., Water Research, 30(11), 2691-2706, 1996.
"Modified Jar Test Studies for Removal a Disinfection By-Products (DBPs) and Color Compounds from Groundwater," Pirbazari, M., Williams, M.D. ,Badriya, B.N., Tu, S.C., and Award, J., in Proceedings of the 1996 North American Water and Environment Congress, American Society of Civil Engineers, Anaheim, California, 1996.
"A Hybrid Membrane Filtration Process for the treatment of Water Contaminated with Petroleum Hydrocarbons," Pirbazari, M., Ravindran, V., Badriya, B.N., Tu, S.C., and Williams, M.D., in Proceedings of 1995 Membrane Technology Conference, pp. 353-363, American Water Works Association, Denver, Colorado, 1995.
"MF-PAC for Treating Waters Contaminated with Natural and Synthetic Organics," Pirbazari, M., Badriyha, B.N., and Ravindran, V., Journal American Water Works Association, 84(12), 95-103, 1992.
Another important research activity centers on advanced oxidation processes (AOPs) for the destruction of synthetic organic chemicals (SOCs) in water, apart from disinfection and deactivation of pathogenic microorganisms. These processes include the application of ozone, hydrogen peroxide, and ultraviolet light, either individually or in combination. These processes are mediated by free-radical reactions, and are becoming increasingly complex from the standpoint of scientific analysis.
Our research group is studying the fundamental aspects of the free-radical reaction mechanisms and reaction kinetics, so that these processes can be made more effective for the degradation of a broad spectrum of organic pollutants in waters and wastewaters, including several pesticides and industrial solvents. The analyses of these processes mainly include the generation of hydroxyl radicals and peroxy radicals, the effect of hydroxyl radical reactions on the breakdown of the SOC molecules, and the potential mechanisms and reaction pathways for degrading these compounds.
The main aspects addressed in this research are the following: the formation of disinfection by-products (DBPs) due to the presence of natural organic matter (NOM), the destruction of SOCs, and the generation of products that constitute assimilable organic carbon (AOC) or biodegradable organic matter (BOM). The DBPs of concern include haloacetic acids, aldehydes and ketones, that will be regulated more stringently by the U.S. EPA in the near future. One aspect of the research will address the formation of DBPs and the trihalomethane formation potential during subsequent chlorination. The SOCs of concern are chlorinated pesticides exemplified by alachlor and helptachlor. We are currently investigating the nature of the attack on the SOC molecules by hydroxyl radicals or other oxidative species. Part of this research activity has been funded by the U.S. EPA.
Relevant Publications/Presentations
"Advanced Oxidation Processes for Removal of Natural Organics and Pesticides from Drinking Water," Badriyha, B., Pirbazari, M., in Proceedings of North American Water and Environment Congress ‘96, ASCE, Washington, DC, 1996.
"Advanced Oxidation Processes for the Destruction of Chlorinated Pesticides in Water," Pirbazari, M., and Ravindran, V., Annual Conference, American Institute of Chemical Engineers, Miami, Florida, November 1992.
"Ozonation/H2O2 Oxidation of the Pesticides Alachlor and Heptachlor in Drinking Water," Badriyha, B., Pirbazari, M., Paper presented at the International Ozone Association Conference, Pasadena, California, March 1992.
"Fate and Transformation of the Pesticide Heptachlor in Water Treatment Processes," Badriyha, B., Pirbazari, M., and Miltner, R.J., Paper presented at the 22nd Annual Meeting of the Fine Particle Society, San Jose, California, July 1991.
"Ozonation, Peroxide, and UV Oxidation for Destruction of Chlorinated Pesticide," Badriyha, B. and Pirbazari, M. (in preparation).
"O3/H2O2/UV Oxidation of Chlorinated Pesticide: Mechanisms and Free Radical Reaction," Badriyha, B. and Pirbazari, M. (in preparation).
Adsorption processes, especially those employing activated carbon, represent a fundamental technology for water purification and industrial wastewater treatment. Bioactive adsorption systems using activated carbon are extensively used because they are efficient and cost-effective in degrading a broad spectrum of organic contaminants, and are capable of meeting higher effluent and water reuse standards. These systems consistently maintain high levels of treatment, and high degree of stability and reliability. Our research has included different reactor configurations such as fixed-bed, expanded-bed and fluidized-bed adsorbers. The economics of bioactive carbon adsorption processes are enhanced by in situ regeneration due to biofilm growth on the adsorbent surface. Major advantages of bioactive carbon adsorbers over conventional biological treatment systems include lower land requirements, lower sensitivity to diurnal variations in flows and concentrations, lower resistance to toxic substances, and superior removal of organic components.
An important component of our research is to optimize the efficiency and economics for large-scale bioactive adsorption systems. Economical design and operation of such systems is a fine art using a combination of mathematical modeling techniques and experimental methods. The modeling provides feed-forward approach to reactor design, while the experiments provide feed-back for model verification and/or refinement. Adsorption and biodegradation parameters are obtained from laboratory-scale experiments, and these parameters are used together with other adsorber characteristics as model inputs for performance forecasting of such systems. Laboratory-scale adsorber experiments provide the means for model verification and potential model refinement. The techniques of dimensional analysis and similitude are subsequently employed for economically upscaling laboratory-scale adsorbers to pilot-scale, and eventually, full-scale systems. These techniques combining experimental and modeling approaches are useful in the performance forecasting, simulation, design, and economic evaluation of the process under a variety of operating conditions. Our research is thus directed at developing the protocol for model verification, reactor design and process upscaling.
Funding for the adsorption/biodegradation projects have been provided by several organizations including the US Environmental Protection Agency, Metropolitan Water District of Southern California, Occidental Corporation, and Aratex Corporation. Financial support has also been provided by the Zumberge Research Innovation Research Fund of the University of Southern California.
Relevant Publications/Presentations
"Predictive Modeling of Bioactive Fluidized Bed and Stationary Bed Adsorbers," Pirbazari, M., Ravindran, V., Kim, S.H., and Badriyha, B.N., Environmental Technology (in press, 1997).
"Activated Carbon Adsorption of Natural and Synthetic Organic Chemicals," pirbazari, M., Ravindran, V., Wong, S.P., and Badriyha, B.N., Scientia Iranica (in press, 1997).
"Modeling of Bioactive Carbon Adsorbers: A Hybrid Weighted Residual-Finite Difference Numerical Technique," Pirbazari, M., Kim, S.H., Ravindran, V., and Badriyha, B.N., Applied Mathematics and Computation, 76(2-3), 99-131, 1996.
"GAC Adsorber Design Protocol for the Removal of Off-Flavors (Geosmin & MIB)," Pirbazari, M., Craig, S., Badriyha, B., Ravindran, V., and McGuire, M.J., Water Research, 27(7), 1153-1166, 1993.
"GAC Adsorber Design for Removal of Chlorinated Pesticides," Pirbazari, M., Badriyha, B., and Miltner, R.J., ASCE Journal Environmental Engineering, 117(1), 80-100, 1991.
High nitrate levels in drinking water sources present a potential risk to public health. Background nitrate concentrations in groundwater are usually low. Most nitrate contamination in groundwater resources can be traced to the excessive application of commercial fertilizers. However, some nitrates are contributed by nitrogen fixation by plants, industrial wastes, domestic wastewaters, landfills, and animal wastes. Generally, nitrate salts reach groundwater by percolation through the soil. Estimates based on data collected in the US Environmental Protection Agency (USEPA) National Pesticides Survey indicate that 5 percent of both public and private drinking water wells have nitrate concentrations greater than the USEPA maximum contaminant level (MCL) of 10 mg NO3-N/L.
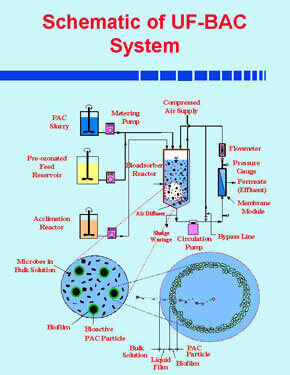
A membrane bioadsorber (MBA) process is employed for denitrification of groundwater. The MBA process is a hybrid technology that amalgamates adsorption, biodegradation and membrane filtration in one unit. Nitate removal is accomplished using a acclimated culture of heterotrophic microorganisms. Removals of pesticide and THM precusors are accomplished by powder activated carbon (PAC). Microfiltration membranes provide a barrier to microorganisms including bacteria and viruses.
The present research focuses on the development of a predictive mathematical model describing the process dynamics of MBA process. The phenomenological aspects of the model encompass the following sub-processes: (a) biological reaction in bulk liquid solution, (b) film transfer from bulk liquid phase to the biofilm-liquid interface, (c) diffusion with biological reaction inside biofilm, (d) adsorption equilibrium occurring at the biofilm-carbon interface, and (e) diffusion within the activated carbon particles
The model exhibited good predictive/simulative capability for nitrate removal. Sensitivity analyses were performed with respect to model parameters including those representing adsorption equilibrium and kinetics, liquid film mass transfer, biofilm transport, biological degradation kinetics, influent concentration, and reactor hydraulic retention time. The model was successful in predicting the steady-state and transient-state process dynamics under a variety of operating conditions.
Reference:
Pirbazari M. et al. (1990) Membrane/Biodegradation Technology for Water and Wastewater Treatment, US Patent No. 4,956,093.
H.H. Tsai, V. Ravindran, and M. Pirbazari, "Model Development for Predicting Process Dynamics of the Ultrafiltration-Biologically Active Carbon (UF-BAPC) System," AIChE Annual Conference, Los Angeles, California, Nov 2000.
M.D. Williams, H.H. Tsai, and M. Pirbazari, "Development of a Predictive Membrane-bioreactor Model for the Removal of Biodegradable Organic Carbon from Water," AIChE Annual Conference, Los Angeles, California, Nov 2000.
H.H. Tsai, V. Ravindra, and M. Pirbazari, "Model Development for the predicting Process Dynamics of Membrane Bioadsorber," In preparation.
A UV Radiation/Hydrogen Peroxide Oxidation Processas a Pretreatment to Nanofiltration for Removal ofNatural and Synthetic Organic Compounds in Water Supplies
The upcoming stringent water quality regulations have raised the necessity to reexamine the efficacy of conventional treatment processes. Nanofiltration is a promising technology to meet prospective regulations in the treatment of NOM in source waters. However, the loss of membrane flux due to fouling is one of the main impediments in the application of membrane processes for drinking water production. Additionally, nanofiltration is ineffective in removing THM precusors, Viruses, pesticides, and hydrogen sulfide from source water. This research is intended to study methods to improve the performance of nanofiltration with respect to permeate flux and quality. In order to accomplish these goals, UV/H2O2 pretreatment of source water prior to nanofiltration as well as membrane cleaning are investigated. In order to mitigate membrane fouling, it is important i) to develop a better understanding regarding the nature of foulants, and ii) to gain insight into the cleaning mechanisms. In this regard, surface characterization of fouled membranes may provide such information.
For the optimization of the UV/H2O2 process, a kinetic model that can predict the decomposition rates of natural and synthetic organic chemicals in different source waters is developed. The model incorporates different water quality parameters (e.g., inorganic carbon concentration and NOM concentration) as well as various operation conditions (e.g., UV intensity, pH, and hydrogen peroxide dosage).
The objectives of this study are summarized below:
- Optimize UV/H2O2 process using an experimentally verified kinetic model for the decompositionof NOM and SOCs.
- Investigate the effect of UV/H2O2 pretreatment on the performance of nanofiltration with respect to permeate flux, permeate quality, and cleanability of fouled membranes.
- Evaluate the effectiveness of three different chemical agents including base, acid and anionic surfactant for the cleaning of NOM-fouled membranes, and investigate their effects on the solute rejection.
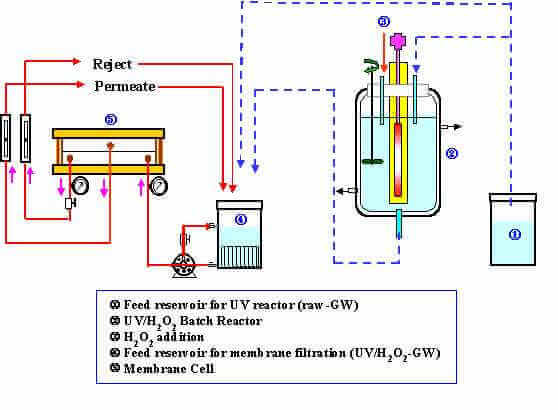
Schematic layout of the UV/H2O2-NF process.
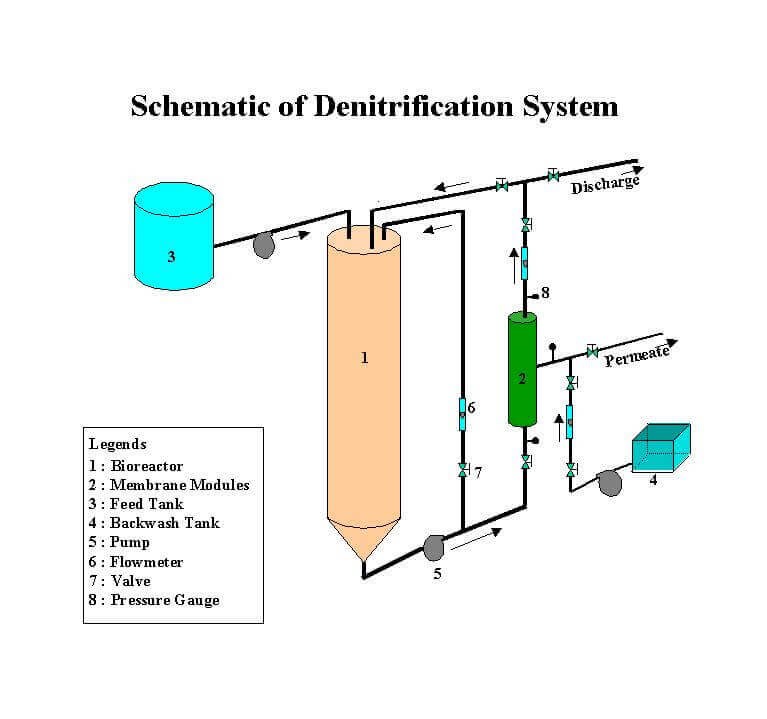
This study investigates the application of anaerobic fluidized bed biofilm reactors (FBBR) to denitrify the brine originating from the reverse osmosis (RO) membrane process at the Water Factory-21 in Orange County Water District, a renown wastewater recycling facility. As the RO processes become widely used as opposed to existing conventional treatment techniques, the discharge regulations pertaining to their toxic by-products such as brine will become more stringent as to force the recycling facilities to take actions on treating it prior to discharge to surface waters.
Brine contains considerably high concentrations of ammonia, salt (total dissolved solids) and heavy metals. Ammonia, which makes up the scope of this study, is a serious treat to aquatic life in the sense that it is toxic to almost all the marine organisms and causes deoxygenation in the system. The impetus for studying the FBBR process are as follows: (1) reduced reactor size due to high biomass concentration, (2) vast surface area for the attachment of biomass on GAC, (3) resiliency of the biomass to process shut-down for maintenance, (4) resistance to shock loads, and (5) lower capital cost as well as easy operation and maintenance.
The objectives of this research are: (i) investigating the efficiency of the FBBR-GAC denitrification process, (ii) studying the effect of influent total dissolved solids concentration on the process efficiency, (iii) optimizing the process efficiency to maximize the economy of the system, and (iv) forecasting the process performance by using mathematical models.
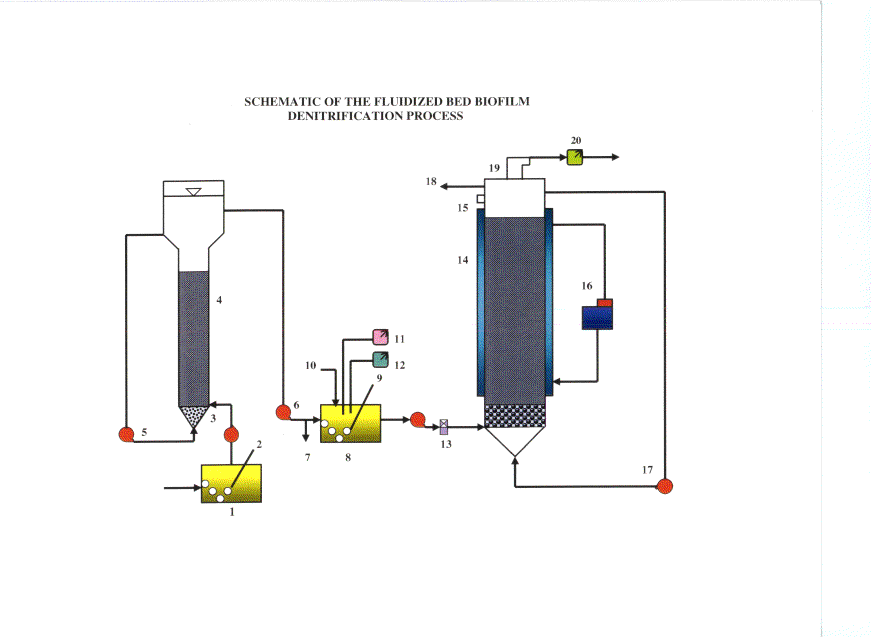
Legends: 1) Brine tank, 2) Nitrogen gas diffuser, 3) Brine influent to nitrification reactor, 4) Nitrification reactor with GAC, 5) Recirculation line, 6) Influent line to denitrification tank, 7) Sampling port, 8) Nitrified brine tank, 9) Air diffuser, 10) Nutrient feed, 11) pH meter, 12) DO meter, 13) Flowmeter, 14) Denitrification reactor with GAC, 15) Sampling port, 16) Water bath, 17) Recirculation line, 18) Denitrified effluent, 19) Nitrogen gas collection channel, and 20) Mass flowmeter for nitrogen gas.
Asphalt is used in a variety of applications, the most significant of which are the paving of roads, highways, and air-fields, as well as the water-proof sealing of tanks, containers, and roofs of buildings. In the United States, the annual asphalt consumption for paving roadways, highways and air-fields is over 25 million tons, and for other applications such as roof-sealing is over 10 million tons. Hot mix asphalt generates emissions of reactive organic gases (ROGs) and particulate matter (PM). As asphalt is a complex mixture, the ROGs emitted encompass a broad spectrum of organic contaminants including several volatile organic compounds (VOCs) and semi-volatile organic compounds (SVOC's) such as aromatics, aliphatics, alicyclics, polynuclear aromatic hydrocarbons (PAHs). The ROGs play an important role in photochemical smog formation and visibility degradation. Also significant in this regard are PM referred to as aerosols, on which ROGs are adsorbed. These aerosols of particle sizes under 10 microns (PM10) or 2.5 microns (PM2.5) are important from an air quality standpoint because they can be irreversibly trapped in the pulmonary tract. Our research is directed at qualitatively evaluating the short-term and long-term asphalt emissions, and estimating the magnitude of the pollution problem. One of our projects in air pollution control was funded by the South Coast Air Quality Management District, with co-funding from the National Science Foundation and the Strategic Highway Research Program (of the Federal Highway Administration) .
The first phase of the project was designed to obtain a priori estimates of PM and ROG emissions from laboratory-scale testing for various types of asphalt and bitumen. Field studies were conducted for evaluating PM and ROG emissions from actual road paving operations including, the hot-mix asphalt preparation, asphalt transportation in trucks, asphalt spreading, and asphalt compaction. Asphalt fractionation studies were conducted to determine the proportions of the characteristic fractions: (a) saturates; (b) naphthene aromatics; (c) polar aromatics; and (d) asphaltenes. Two of these generic asphalt fractions, namely, polar aromatics and naphthene aromatics contain significant quantities of PAHs, VOCs and SVOCs. The fractionation studies assisted in the identification of potential ROGs that would be emitted from hot asphalt. These experiments employed asphalt types that are currently used for road paving in California, as well as bitumen used for roof-sealing operations.
An important component of this research was the development of an appropriate protocol for the testing, sampling and analysis of PM and ROG emissions from asphalt. These procedures were developed for the quantification of PAHs, VOCs and SVOCs at trace levels as well as PM. The analytical techniques for PAHs involved high performance liquid chromatography (HPLC) with fluorescence detection, and gas chromatography (GC) with flame ionization detection. The emissions of VOCs and SVOCs were analyzed by GC techniques. Gas chromatography/mass spectrometry (GC/MS) methods were used for the identification of the organic contaminants emitted from hot asphalt mix. A quality assurance and quality control was developed and strictly observed throughout this work.
This research also evaluated the effect of additives on the PM and ROG emissions from hot asphalt. The study compared various classes of emissions from rubberized asphalt and with those from regular asphalt. The motivation behind these investigations was to observe whether rubberization of asphalt for improving the mechanical properties by matrix modification would result in increase or decrease in the overall particulate and organic emissions. Furthermore, this project included an initial study on the potential for photochemical transformation of PAHs, VOCs and SVOCs in the atmosphere. Investigation of potential photochemical transformation products from asphalt emissions is an ongoing project.
It was postulated that the actual emissions from road-paving operations could be two orders of magnitude higher than the estimates obtained from our field sampling studies because field samples were diluted and dispersed by wind effects. In order to obtain realistic quantitative estimates, laboratory simulation studies of road-paving operations were conducted during the second phase of the project. This provided realistic quantitative determination of ROG and PM emissions from hot asphalt. This information proved important for estimating the potential for photochemical smog formation and adverse health effects. The research findings was useful for regulating agencies such as the U.S. Environmental protection Agency, Air Resources Board, and the South Coast Air Quality Management District to assess the magnitude of the problem so that they can suggest appropriate control and mitigation measures. The results also provide sufficient incentive for the Federal Highway Administration to develop new asphalt additives that could potentially reduce hazardous emissions.
Relevant Publications/Presentations
Badriyha, B.N. Kitto, A.M., Ravindran, V., Tyner, R., Pirbazari, M., and Synolakis, C.E., "Evaluation of Hazardous Emissions from Hot-Mix Asphalt: Field Studies for Risk Assessment." Paper presented at the Annual Meeting of the American Institute of Chemical Engineers, San Francisco, CA, November 1994.
"Emission of Organic Pollutants from Asphalt," First Annual Report, submitted to the South Coast Air Quality Management District, California, 1994.
"Protocol for the Testing, Sampling, and Analysis of Asphalt Emissions," Technical Report, submitted to the South Coast Air Quality Management District, California, 1994.
Badriyha, B.N., Pirbazari, M., Ravindran, V., Kitto, A.M., and Synolakis, C.E. "Emission of Volatile and Semi-Volatile Organic Compounds from Hot Asphalts in Laboratory -Scale and Field Studies." Paper presented at the Conference on Measurement of Toxic and Related Air Pollutants under the auspices of the Air and Waste Management Associatio, U.S. Environmental Protection Agency, Research Triangle Park, NC, 1996.
"Emission of Organic Pollutants from Asphalt," Final Project Report, submitted to the South Coast Air Quality Management District, California, 1996.
Kitto, A.M., Pirbazari, M., Badriyha, B.N., Ravindran, V., Tyner, R. and Synolakis, C.E. "Emissions of Volatile and Semi-Volatile Organic Compounds and Particulate Matter from Hot Asphalts." Environmental Technology, 18(2), 121-138 (1997).
"Development of Experimental Methodologies for the Assessment of Organic Emissions from Asphalt," Pirbazari, M, with Kitto, A. M., Badriyha, B.N., Ravindran, V., and Synolakis, C.E., Environmental Science and Technology (under preparation).
"Chromatographic Trace Analysis of Polynuclear Aromatic Hydrocarbon Emissions from Hot Asphalt," Pirbazari, M, with Kitto, A. M., Badriyha, B.N., Ravindran, V., and Synolakis, C.E., Journal of Chromatography (under preparation).
Although biofilters have been widely used for odor control for more than four decades, itsapplication for treating toxic volatile organic compounds (VOCs) has only started in recent years. Stringent regulatory requirement forces both POTW and industrial communities to explore potential technologies that are more cost effective, yet efficient enough to comply with the regulation. However, a major factor that hinders the extended applications of biofiltration technology is the biodegradability of the organic pollutants. Thus, to effectively remove the very slowly biodegradable VOCs, a forefront challenge is hereinafter encountered to optimize the biofilter performance.
Chlorinated VOCs, such as methylene chloride and trichloroethylene, which are known carcinogens, are among the most recalcitrant air pollutants. Although relatively low concentrations of these compounds (methylene chloride: 6-350 mg/L; trichloroethylene: 1-25 mg/L) have been reported in typical wastewater treatment facilities, significant emissions are reportedin chemical, coating and electronic industries (approximately 75 million pounds of methylene chloride and 30 million pounds of TCE released in U.S. during 1993). Relatively few research efforts have been focused on removal of methylene chloride through a biofilter, and even less information are available in treating TCE with biofiltration. While successful removal of methylene chloride has been reported in the literature, only partial removal for TCE has been documented. It has been well known that aerobic degradability of chlorinated hydrocarbons decreases with increasing number of chlorine, however the reversed trend is found true in anaerobic biodegradation. Nevertheless, anaerobic biofilter treating gaseous phase pollutant are deemed impractical since pretreatment would be required to eliminate oxygen from the contaminated air.
Two bench-scale aerobic biofilter columns treating binary component (methanol and methylene chloride vapor) have been operative in the Environmental Engineering Laboratories of the University of Southern California for more than two years. The two columns, which are identical in size, were packed with either pelletized activated carbon (GAC) or granular anthracite to study the effect of an adsorbing medium versus a non-adsorbing medium. In addition, a third aerobic column packed with activated carbon and inoculated with a pure strain of bacteria was operated for ten months to compare the capability of a pure culture vis-à-vis a mixed consortium in the biodegradation of methylene chloride. Better than 99 percent removal efficiency was consistently achieved.
Anaerobic biofilters were also investigated to assess their efficiencies in removing methylene chloride.
Biofilter Modeling and Design
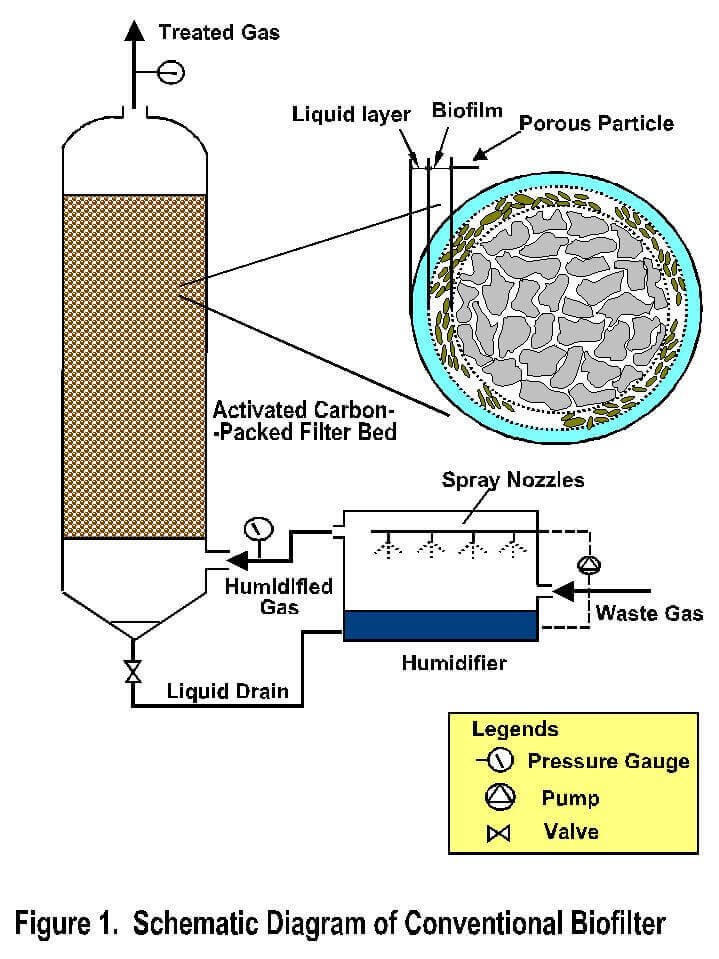
A traditional approach for the design of biofilters would require bench-scale or laboratory-scale feasibility studies for the treatment of specific gas stream, followed by pilot-scale studies, and eventually full-scale design. However, this procedure could prove expensive in view of the time, effort and cost associated with pilot-scale studies. On the other hand, development of a biofilter model would serve as a useful tool in predicting the performance of biofilters in real situations, or simulating their dynamics under a variety of operating conditions. A biofilter model has been developed specifically for this purpose, incorporating all the phenomenological processes and sub-processes occurring in a real biofilter. These phenomena include the following: transport of contaminants from the gas phase into liquid film, sorption of contaminants on media particles, and biodegradation of contaminants within the biofilm. The biofilter modeling and design protocol adopted by our research group has been successfully used to forecast the performance of several pollutants including cholrinated VOC's.
Our biofilter model employs several input parameters such as adsorption and biodegradation constants, obtained from independent bench-scale studies, as well as system conditions including flow rate, biofilter dimensions, media characteristics, etc. This feed-forward approach provides the means for proper design of laboratory- scale biofilters. Results from laboratory- scale adsorber experiments will provide the necessary feedback to evaluate the predictive capability of the model, and to implement potential model refinements whenever necessary. The modeling protocol also involves process up-scaling from laboratory- to pilot- , and eventually to full- scale design.
Relevant Publications/Presentations
Den, W., Pirbazari, M., Huang C.C., and Shen K.P. "Technology Review for Vapor Phase Biofiltration. Part I: Technological Development and Applications.", Journal of the Chinese Institute of Environmental Engineering, 8(3), pp. 159-179 (1998).
Den, W., Pirbazari, M., Huang C.C., and Shen K.P. "Technology Review for Vapor Phase Biofiltration. Part II: Biofilter Design and Operation.", Journal of the Chinese Institute of Environmental Engineering, 8(3), pp. 181-196 (1998).
Pirbazari, M., and Den, W. "Microbial Ecology of Biofiltration" Invited Presentation at Westminster University, Department of Biology, London, U.K., August 15, 1997.
Den, W., and Pirbazari, M. " Biofiltration: An Emerging Air Pollution Control Technology" Presented at the Hazardous Waste III Conference, Los Angeles, CA, October 23, 1997.
Den, W., Badriyha, B., and Pirbazari, M., "Biofiltration of Methylene Chloride: Aerobic and Anaerobic Absorbers", (to be submitted).
Den, W., and Pirbazari, M., "Modeling and Design Protocol for the Biofiltration of Volatile Organic Compounds", (in preparation).
Den, W., and Pirbazari, M., "Biofilm Degradation of Vapor-Phase Trichloroehtylene from Industrial Discharges", (in preparation).
Man M.K., Den,W., Badriyha, B. and Pirbazari, M., "Biofiltration for the Removal of Volatile Organic Compounds" presented at the 1996 North American Water Environment Conference, ASCE, Anaheim, CA, June 25, 1996.
Den, W., and Pirbazari, M. " Biofilters for Treating Air Streams Contaminated with Chlorinated VOCs", Presented at the Industrial Technology Research Institute, Union Chemical Laboratory, Hsin Chu, Taiwan, May 29, 1997.
Den, W. "Biofiltration for the Treatment of Chlorinated Volatile Organic Compounds in Industrial Emissions: Modeling and Design Protocol," Doctoral dissertation in progress, Department of Civil and Environmental Engineering, University of Southern California, Los Angeles.
Man, M.K. "An evaluation of Packing Media for the Biofiltration of Methylene Chloride and Methanol Vapors," Master of Science thesis, Department of Civil and Environmental Engineering, University of Southern California, Los Angeles, December 1995.
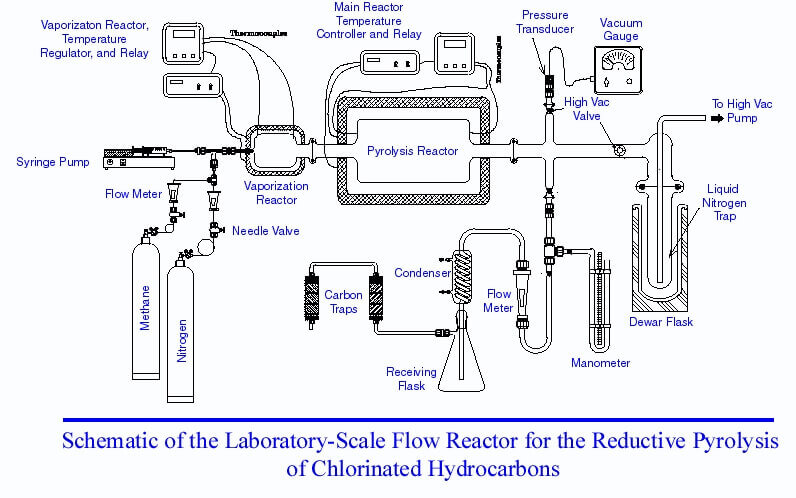
Thermal technologies such as incineration, combustion and catalytic dehalogenation have been widely used for the destruction of hazardous wastes. Although these technologies are effective for the destruction of non-halogenated hazardous wastes, they have problems in the case of halogenated hydrocarbons. The reductive pyrolysis technology developed at USC has been demonstrated to be effective in achieving complete destruction of halogenated wastes exemplified by polychlorinated biphenyls (PCBs) and chloromethane.
The reductive pyrolysis technology may have the potential of replacing conventional combustion processes in future, because the latter often lead to the formation of undesirable byproducts such as dioxins and dibenzofurans, regarded as serious air pollutants. Furthermore, catalytic dehalogenation technologies often encounter the problems of catalytic poisoning and deactivation. The pyrolytic process employs a reductive environment to pyrolyze the halogenated hydrocarbons to yield simpler non-halogenated hydrocarbons and the corresponding hydrogen halide. In the case of PCBs, the reaction products are simpler non-halogenated hydrocarbons and hydrogen chloride. Our research group has investigated the reductive pyrolysis of PCBs, and the free-radical reaction mechanisms and pathways associated with the process. The thermodynamics of the process for destroying chlorinated hydrocarbons was investigated from the standpoint of bond-energy considerations, to establish the reasoning behind the process feasibility to achieve complete destruction (over 99.9999 percent). Results were obtained in batch as well as continuous flow reactor systems demonstrating the high destruction efficiency of the process. The kinetics of the free-radical reactions were analyzed on the basis of radical chain mechanisms associated with hydrogen atoms, and methyl, alkyl as well as aryl radicals. The thermokinetic properties such as the Arrhenius factors and activation energies were estimated to evaluate the overall process kinetics. This research was initially funded by the California Department of Health Services, and was performed in collaboration with the USC Loker Hydrocarbon Research Institute.
Relevant Publications/Presentations
"Pyrolytic Destruction of Polychlorinated Biphenyls in a Reductive Atmosphere," Evans, D.H., Pirbazari, M., Benson, S.W., Tsotsis, T.T. and Devinny, J.S., Jour. Haz. Mat., 27, 253-272, 1991.
"Reductive Pyrolysis for the Destruction of Chlorinated Hydrocarbons," Pirbazari, M., Ravindran, V., Benson, S.W., Badriyha, B.N., and Evans, D.H., Combustion Science and Technology, 122(1-6), 183-213, 1997.
"Thermal Destruction of Chlorinated Hydrocarbons: Thermokinetic Considerations," Pirbazari, M., Benson, S.W., Ravindran, V., and Badriyha, B., Annual Conference, American Institute of Chemical Engineers, San Francisco, California, November 13-18, 1994.
Reductive Pyrolysis for the Destruction of Chloromethane: Reaction Pathway Model Based on Thermodynamic and Thermokinetic Considerations," Pirbazari, M., Benson, S.W., Ravindran, V. and Badriyha, B., North American Water and Environment Congress '96, ASCE, Anaheim, California, June 22-28, 1996.
Free-Radical Reaction Mechanisms and Pathways for the Reductive Pyrolysis of Polychlorinated Biphenyls," Pirbazari, M., Benson, S.W., Badriyha, B., Ravindran, V. and Evans, D.H., Annual Conference, American Institute of Chemical Engineers, St. Louis, Missouri, November 1993.

